|
|
|
Medical
Supplier Inspection &QC
|
|
This page is about importers and exporters of Supplier Inspection &QC Search in a category : Medical Search in a category : supplier, inspection |
Thursday, March 02, 2017
Quantity : 50
Product Description Stainless Steel COMMUNION WARE / CHURCH PRODUCTS We offer exclusive range of Communion Ware in high quality of Stainless Steel in smooth matte finish. We manufacture all these products in high quality Stainless Steel in 0.60 mm thickness. Our Communion Ware...
Mayur Exports
- mayur3559
- 342003 - Jodhpur
- +91 291 2742924
Thursday, January 18, 2024
Price : 13955.57-697778.50 U
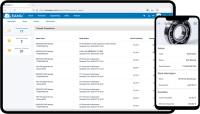
Know everything about your spare parts with our maintenance parts inventory software! With EAMic® spare parts management system software, you can easily check suppliers' references, technical characteristics, consumption, storage box code, etc. You won't miss anything on your spare...
- 200030 - shanghai
- 021 6958 0696
Friday, June 14, 2019
Quantity : 500 - Price : 45,99 €
Lit d'invité Pliable a la Taille 80X200 cm, Structure en métal, avec roulettes, Matelas H. 11 cm, 80 x 200 cm Inclus Lit d'invité Pliable a la Taille 80X200 cm, Structure en métal, avec roulettes, Matelas H. 11 cm, 80 x 200 cm Inclus - Lit d'invité pliable Bflyshop est extrêmement confortable...